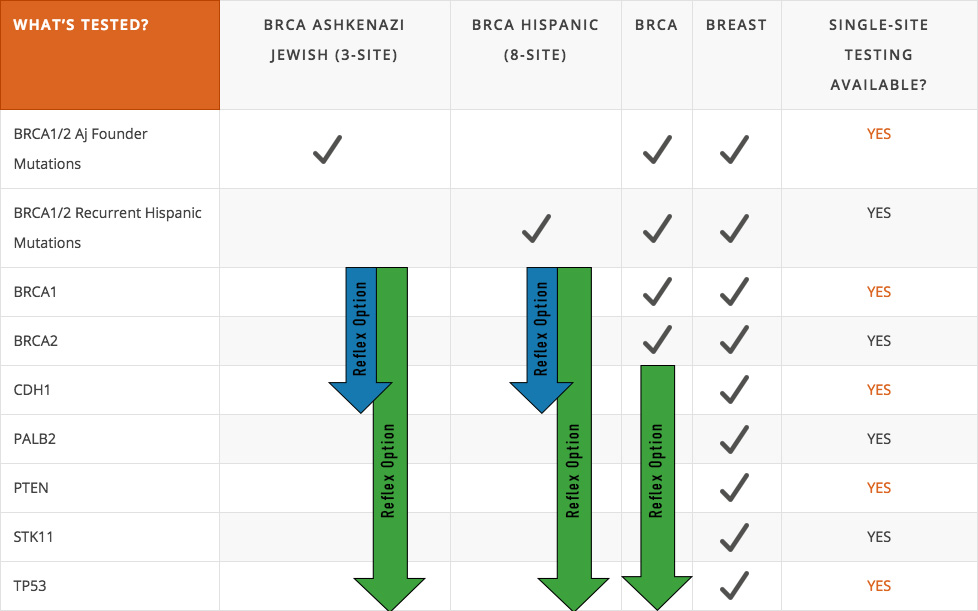
BRCA1 description
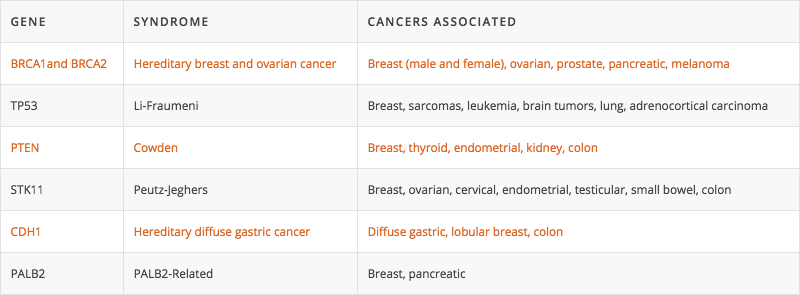
Pathogenic variants in the BRCA1 gene are associated with hereditary breast and ovarian cancer (HBOC) syndrome, and convey a significantly increased risk of both breast and ovarian cancer. Studies have demonstrated that BRCA1 gene pathogenic variants can be associated with up to an 85% breast cancer risks and up to a 63% ovarian cancer risks. Furthermore, women with a BRCA1 pathogenic variant have an increased risk of a second breast cancer diagnosis (contralateral or ipsilateral). This risk has estimated to be up to 27% within the first 5 years of diagnosis and up to 40-50% within 20 years of initial diagnosis. Pathogenic variants in BRCA1 may also be associated with an increased risk of other types of cancer, including peritoneal, pancreatic, prostate, and male breast cancer. However, the exact level of risk is not yet known.
BRCA2 description
Pathogenic variants in the BRCA2 gene are associated with Hereditary Breast and Ovarian Cancer (HBOC) syndrome, and convey a significantly increased risk of several cancers, including breast, ovarian, pancreatic, melanoma, and prostate cancer. Studies have demonstrated that BRCA2 gene pathogenic variants can be associated with up to an 84% breast cancer risk and up to a 27% ovarian cancer risk. Furthermore, in women who have had breast cancer, having a BRCA2 pathogenic variant increases risk of a second breast cancer diagnosis (contralateral or ipsilateral). This risk has estimated to be up to 12% within the first 5 years of diagnosis and up to 40–50% within 20 years of initial diagnosis. BRCA2 pathogenic variants may also be associated with small increased risks of developing primary peritoneal, gallbladder, bile duct, and stomach cancer. However, these risks are not yet clearly defined.
Inheritance and Familial Implications
BRCA1 and BRCA2 pathogenic variants are inherited in a dominant pattern, meaning that anyone with a pathogenic variant has a 50% chance of passing it on to each of his or her children. Conversely, this also means that they have a 50% chance of not passing on the pathogenic variant to each child. Individuals who carry a BRCA1 or BRCA2 pathogenic variant do not have any control over whether or not a variant is passed on to their children. Both men and women can have pathogenic variants in BRCA1 or BRCA2 and can pass them down to their children, even though the cancer risks may be different for males and females. In addition to children, each first-degree relative (parents and full siblings) of someone with a BRCA1 or BRCA2 pathogenic variant has a 50% chance to have the same pathogenic variant.
Genetic testing is available to at-risk family members to determine if they also carry the identified BRCA1 or BRCA2 pathogenic variant. Because of the increased risks for certain cancers, identified carriers of the BRCA1 or BRCA2 should seek recommendations from their provider regarding future medical management. Those individuals found not to carry the familial BRCA1 or BRCA2 gene pathogenic variant will have risks similar to the general population.